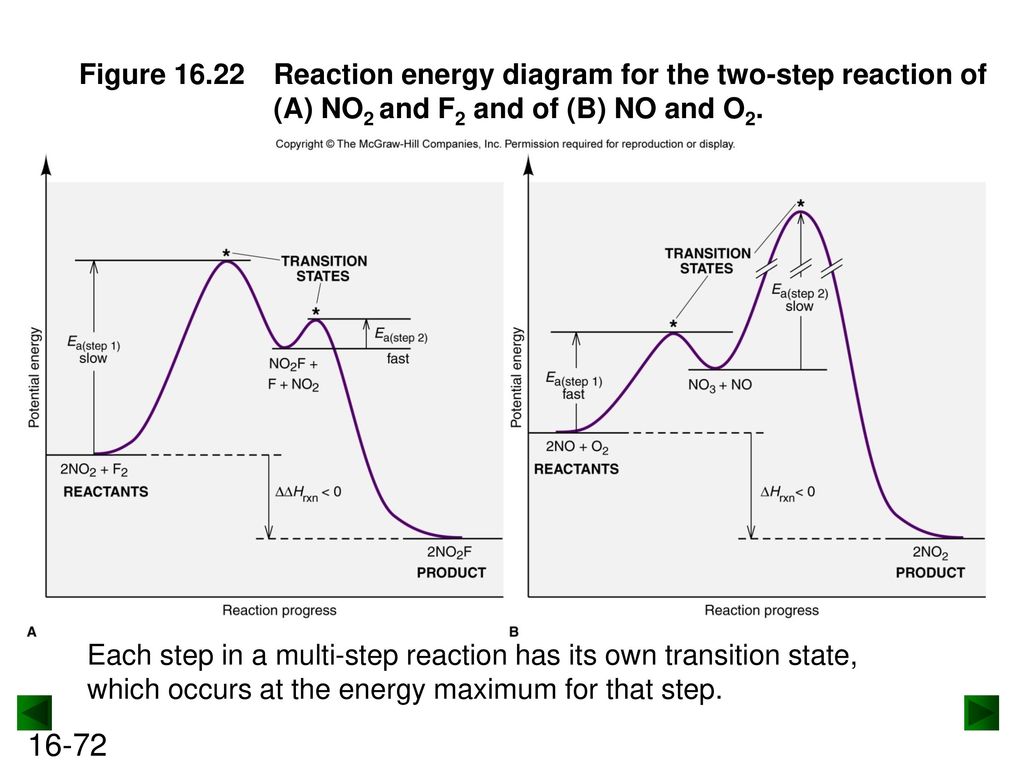
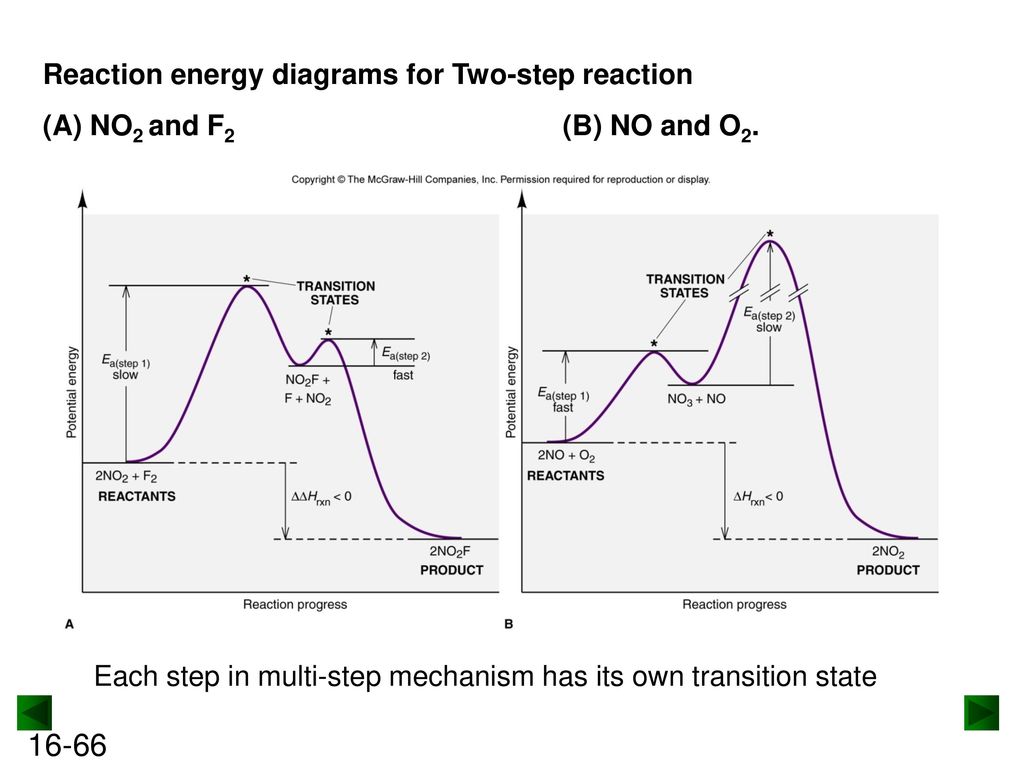
39 energy diagram for a two step reaction
Solved Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer SOLVED: Draw an energy diagram for a two-step exergonic reaction whose ... Exurb you nick to step exactly unique reaction. And it is given that the second step is faster than first to. Download the App! Get 24/7 study help with the Numerade app for iOS and Android! Enter your email for an invite. Sent to: ... Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step ...
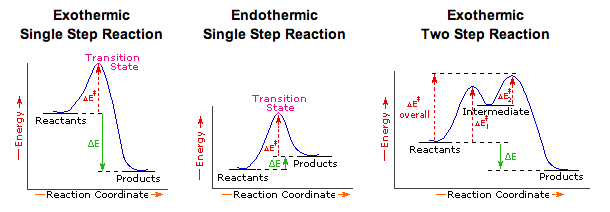
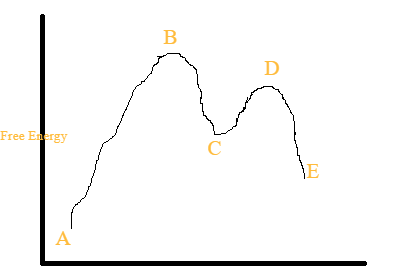
6.8: Energy Diagram for a Two-Step Reaction Mechanism A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise Draw structures representing TS1 and TS2 in the reaction above.
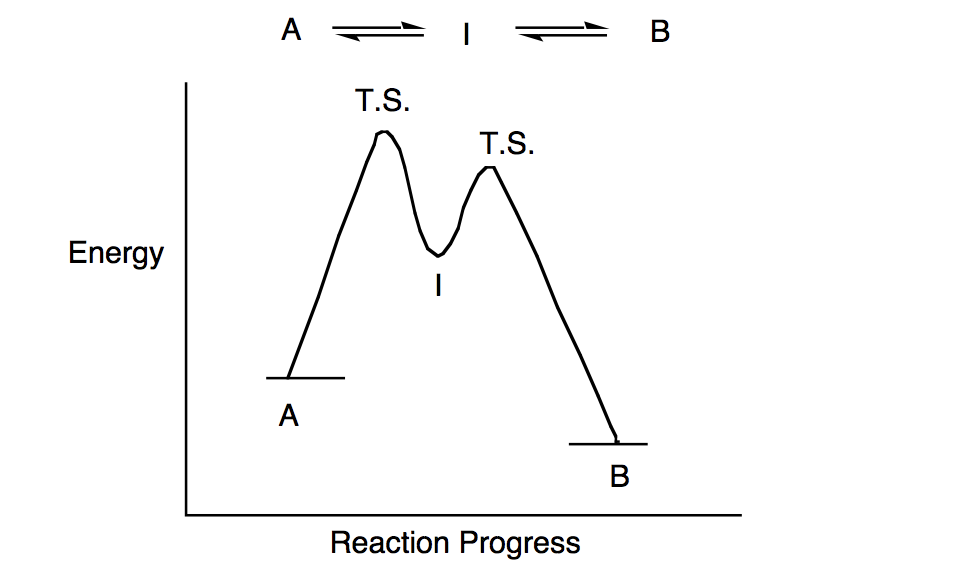
Energy diagram for a two step reaction
Explain the Energy Diagram for a Two-Step Reaction Mechanism Solution for Explain the Energy Diagram for a Two-Step Reaction Mechanism ? 6.7: Reaction Coordinate Diagram for a Two-Step Reaction Mechanism ... A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise Draw structures representing TS1 and TS2 in the reaction above. Draw a reaction-energy diagram for a two-step endothermic reaction with ... Calculate ΔH0 values for the possible reactions of iodine with species present in the chlorination of methane and use these values to explain why iodine inhibits the reaction. (The I-Cl bond-dissociation enthalpy is 211 kJ/mol or 50 kcal/mol). (a) Draw an approximate reaction-energy diagram for the acid-base reaction of phenol (see below) with ...
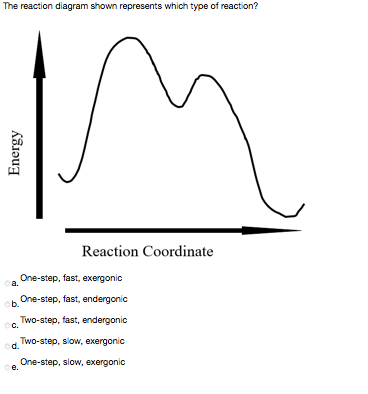
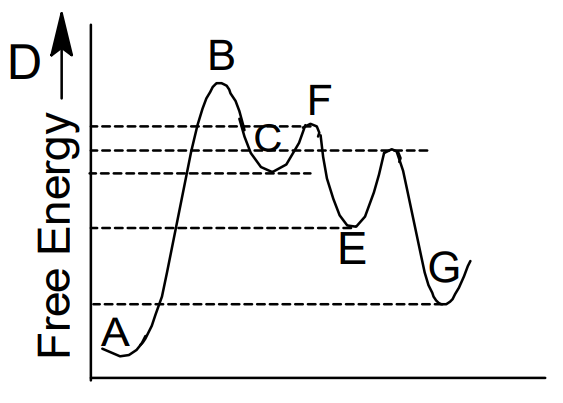
Energy diagram for a two step reaction. Sketch a reaction energy diagram for a two-step react… - SolvedLib Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall $\Delta G^{\dagger},$ and overall $\Delta G^{\circ}.$. 3. Draw the energy diagram for a two-step reaction. | Quizlet Draw the energy diagram for a two-step reaction. | Quizlet Expert solutions Question Draw the energy diagram for a two-step reaction. Solution Verified Create an account to view solutions By signing up, you accept Quizlet's Terms of Service and Privacy Policy Continue with Google Continue with Facebook Sign up with email Solved Choose the energy diagram for a two-step reaction, A - Chegg Choose the energy diagram for a two-step reaction, A → B → C, in which the relative energy of the compounds is A< C < B, and the step A→ B is rate-determining. Select the single best answer. Reaction coordinate Reaction coordinate IV. Reaction coordinate Reaction coordinate II O IV References eBook & Resources Multipart Answer Difficulty: Medium Sketch an energy diagram for a two-step reaction that is endothermic in ... A two-step reaction has two transition states and one intermediate. The intermediate corresponds to the product of the first step and the reactant for the. ... Sketch an energy diagram for a two-step reaction that is endothermic in the first step, exothermic in the second step, and exothermic overall. ...
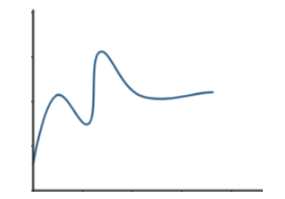
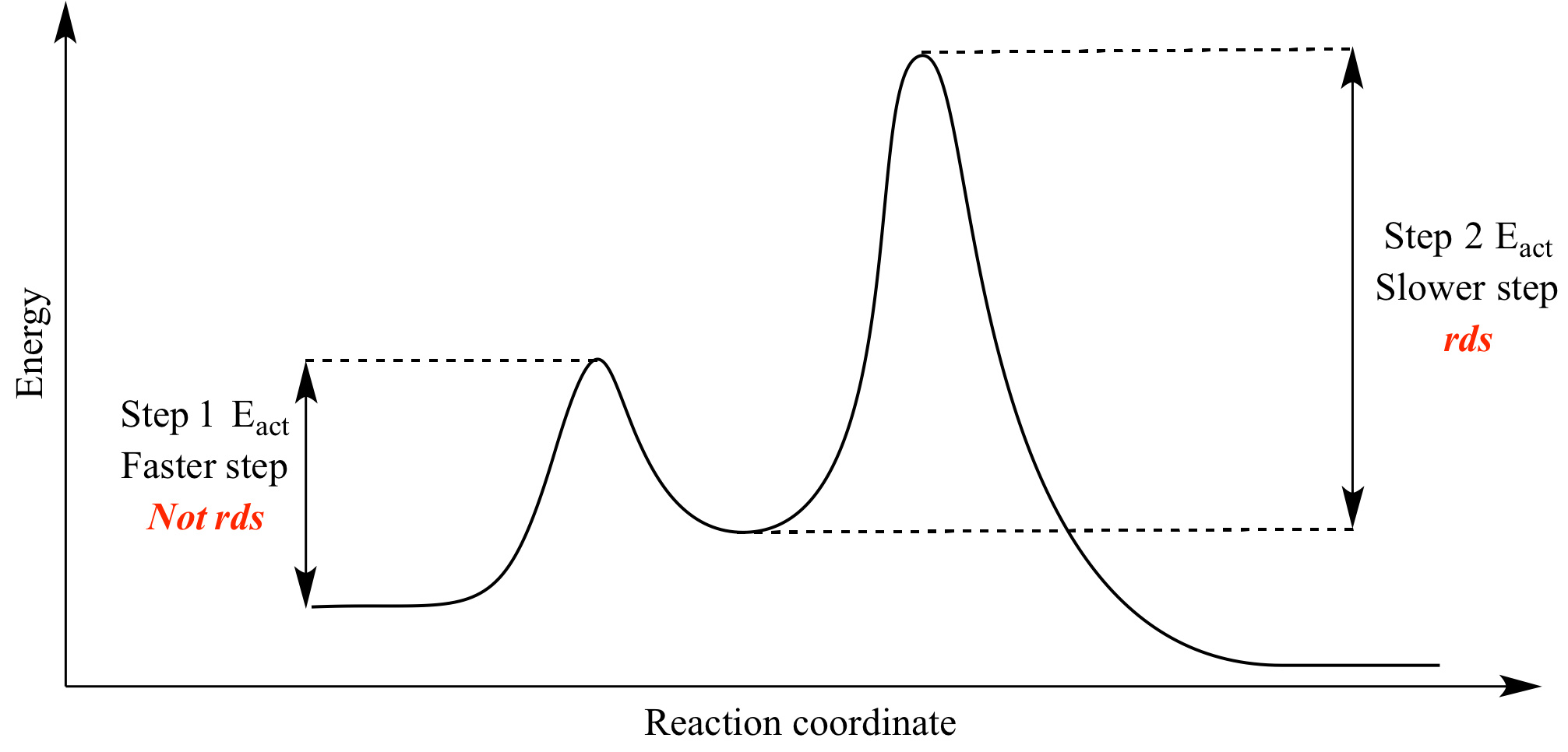
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Draw an energy diagram for a two-step exergonic reaction who | Quizlet Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states. Draw an energy diagram for a two-step exergonic reaction ... - Numerade SOLVED:Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step. VIDEO ANSWER: in this question. We're going to draw the energy during um for two step reaction where the second step of the reaction is faster than the first step of the reaction. It's no. Let's recall the definiti Download the App! Draw a reaction-energy diagram for a two-step endothermic reaction with ... Calculate ΔH0 values for the possible reactions of iodine with species present in the chlorination of methane and use these values to explain why iodine inhibits the reaction. (The I-Cl bond-dissociation enthalpy is 211 kJ/mol or 50 kcal/mol). (a) Draw an approximate reaction-energy diagram for the acid-base reaction of phenol (see below) with ...
6.7: Reaction Coordinate Diagram for a Two-Step Reaction Mechanism ... A potential energy diagram for an S N 1 reaction shows that the carbocation intermediate can be visualized as a kind of valley in the path of the reaction, higher in energy than both the reactant and product but lower in energy than the two transition states. Exercise Draw structures representing TS1 and TS2 in the reaction above. Explain the Energy Diagram for a Two-Step Reaction Mechanism Solution for Explain the Energy Diagram for a Two-Step Reaction Mechanism ?

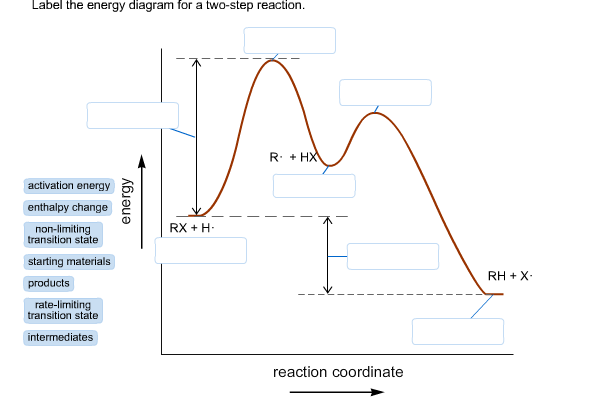



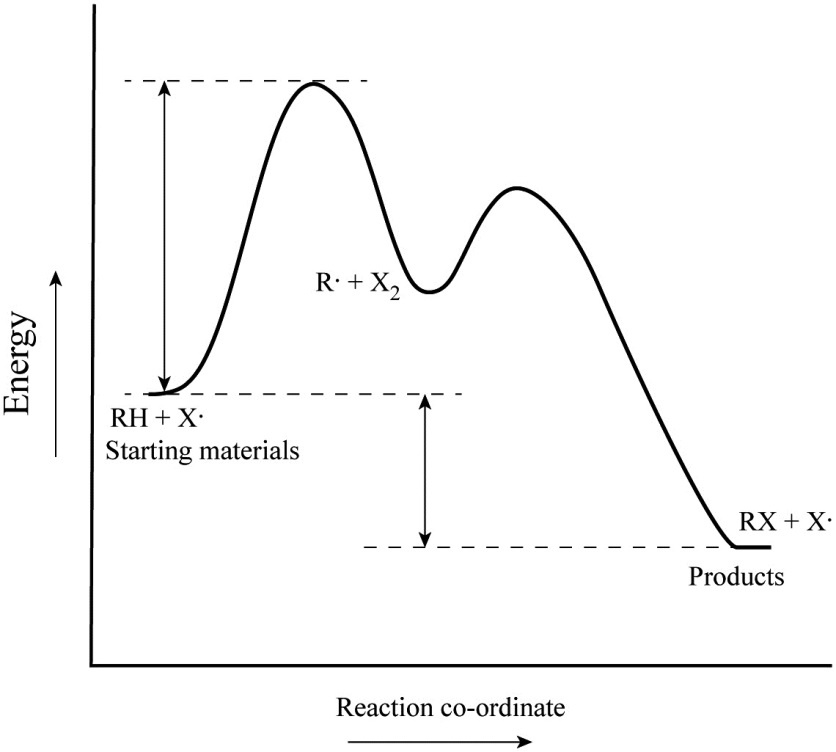



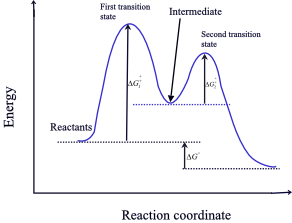
/chapter9/pages3and4/page3and4_files/addition_energy_diagram.png)













Post a Comment for "39 energy diagram for a two step reaction"