44 so2 pi bonds
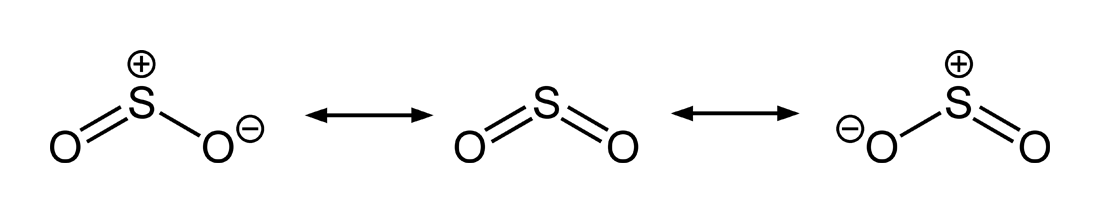
SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY Nov 20, 2019 ... In SO2, the central atom S is double bonded to each O. There are two double bonds in the structure. You know that there can only be one sigma ... The pi bonds in SO2 molecule arises due to overlap. - Doubtnut Jun 27, 2022 ... The pi bonds in SO2 molecule arises due to overlap.
How many pi bonds are in $S{{O}_{2}}$? - Vedantu As we can see that, each atom is connected by at least one single bond which will be counted as $\sigma$ bonds and the rest are $\pi$ bonds. ... Hence there are 2 ...

So2 pi bonds
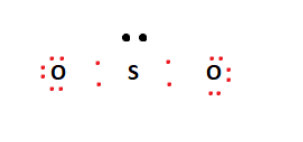
How many pi bonds are in SO_2? - Socratic Mar 27, 2016 ... There are two π bonds in a single SO2 molecule. Explanation: First, let us consider the structure of the SO2 molecule: Source: Wikipedia. Number of bonds in SO2 : | Chemistry Questions - Toppr The sulphur dioxide molecule contains 2σ, 2π bonds and one lone pair of electrons. S atom contains 6 valence electrons out of which it shares 2 electrons ... How does SO2 have 2 π bonds? [duplicate] May 19, 2017 ... The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds ...
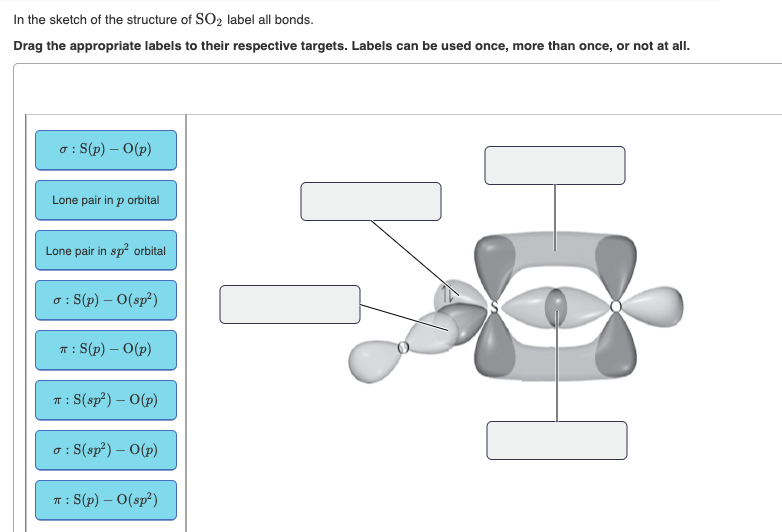
So2 pi bonds. Number of bonds in SO2 are - Doubtnut Jun 27, 2022 ... In angular structure of SO2, the sigma bond between S-O is formed by. 121615083. Text Solution. doubtnut_header_logo_white_new. The π bonds ... SO2 Hybridization - YouTube Aug 13, 2013 ... A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. Hybridization of SO2 (Sulphur Dioxide) - Byju's Dec 25, 2019 ... During the formation of SO2, this central atom is bonded with two oxygen atoms and their structure can be represented as O=S=O. As for the ... SO2 Hybridization (Sulfur Dioxide) - YouTube May 10, 2022 ... It is a chemical formula for Sulfur Dioxide. To find... ... Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3.
How does SO2 have 2 π bonds? [duplicate] May 19, 2017 ... The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds ... Number of bonds in SO2 : | Chemistry Questions - Toppr The sulphur dioxide molecule contains 2σ, 2π bonds and one lone pair of electrons. S atom contains 6 valence electrons out of which it shares 2 electrons ... How many pi bonds are in SO_2? - Socratic Mar 27, 2016 ... There are two π bonds in a single SO2 molecule. Explanation: First, let us consider the structure of the SO2 molecule: Source: Wikipedia.






















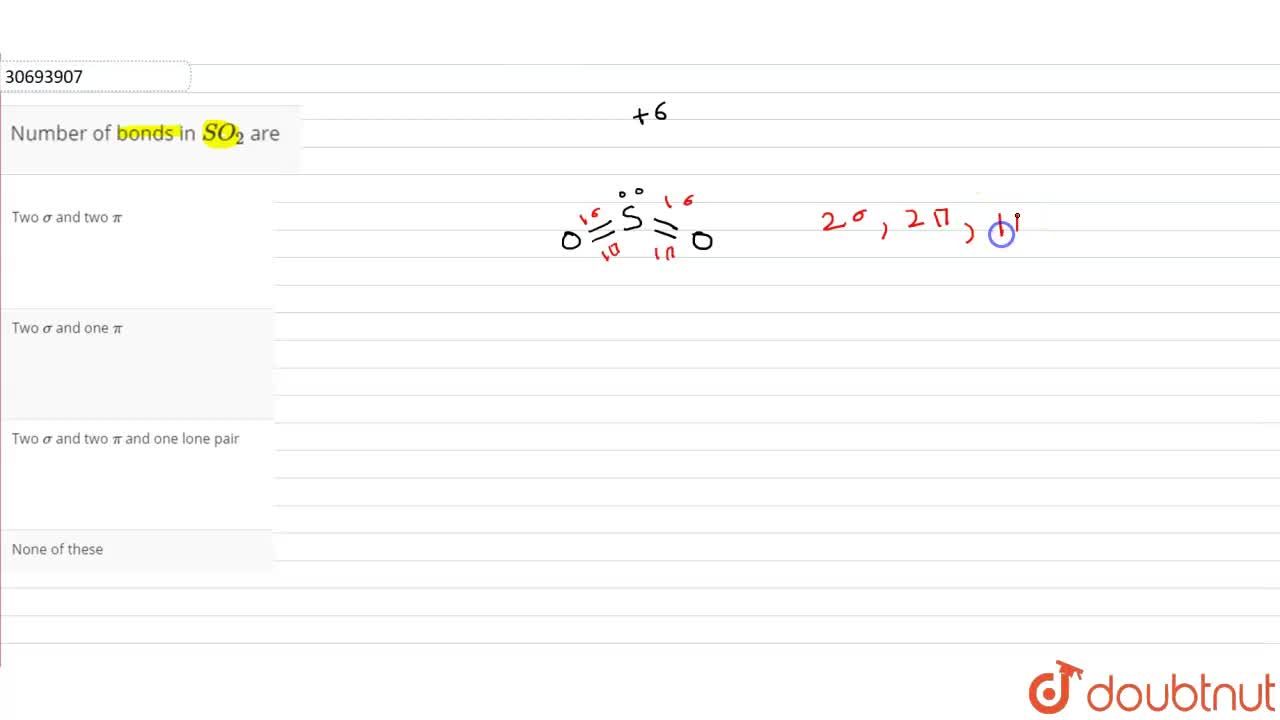
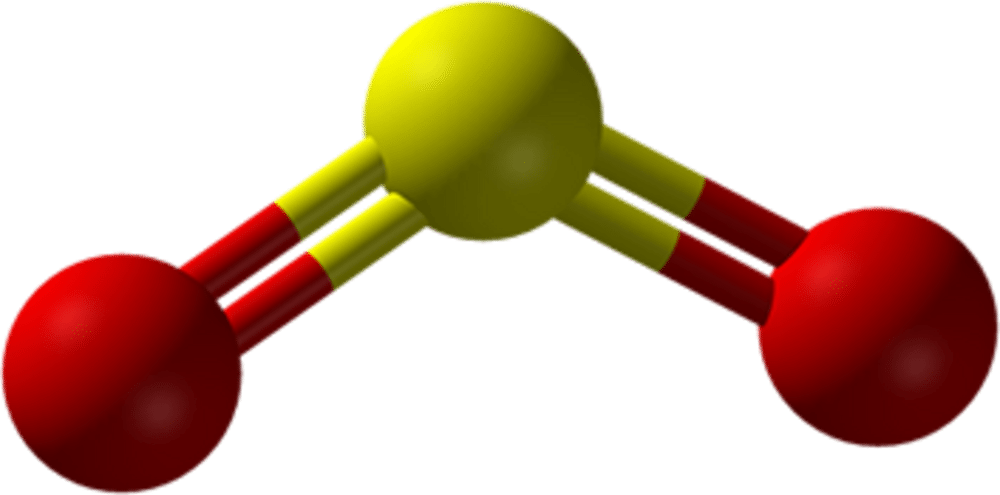


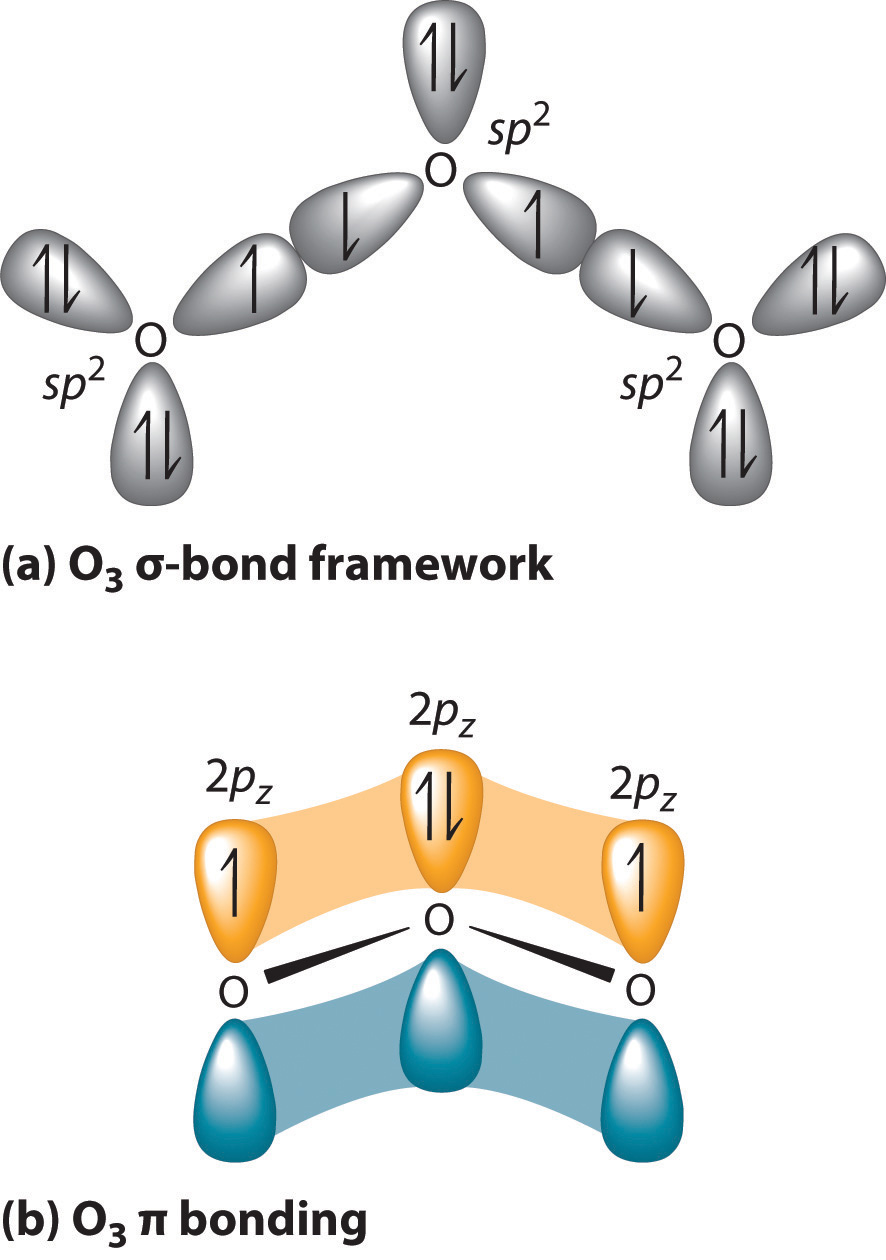

Post a Comment for "44 so2 pi bonds"