42 ndc 27808-065-02
Teva Metformin Recall | DiabetesTalk.Net Prescription Antibiotic Recalled Morton Grove Pharmaceuticals Inc. is recalling 7332 bottles of amoxicillin and clavulanate potassium for oral suspension, USP, 250/62.5 mg per 5 mL, 100 mL when reconstituted (NDC 60432-065-00). NDC 27808-065 Promethazine Hydrochloride And Codeine Phosphate NDC 27808-065-02 . Package Description: 473 mL in 1 BOTTLE, PLASTIC . Price per Unit: $0.04173 per ML. NDC Product Information. Promethazine Hydrochloride And Codeine Phosphate with NDC 27808-065 is a human prescription drug product labeled by Tris Pharma Inc. The product's dosage form is solution and is administered via oral form.
Promethazine Hydrochloride and Codeine Phosphate 产品ndc ndc包装代码 包装描述 药品说明书; 27808-065: 27808-065-02: 473 ml in 1 bottle, plastic (27808-065-02) 20180312 n n:
Ndc 27808-065-02
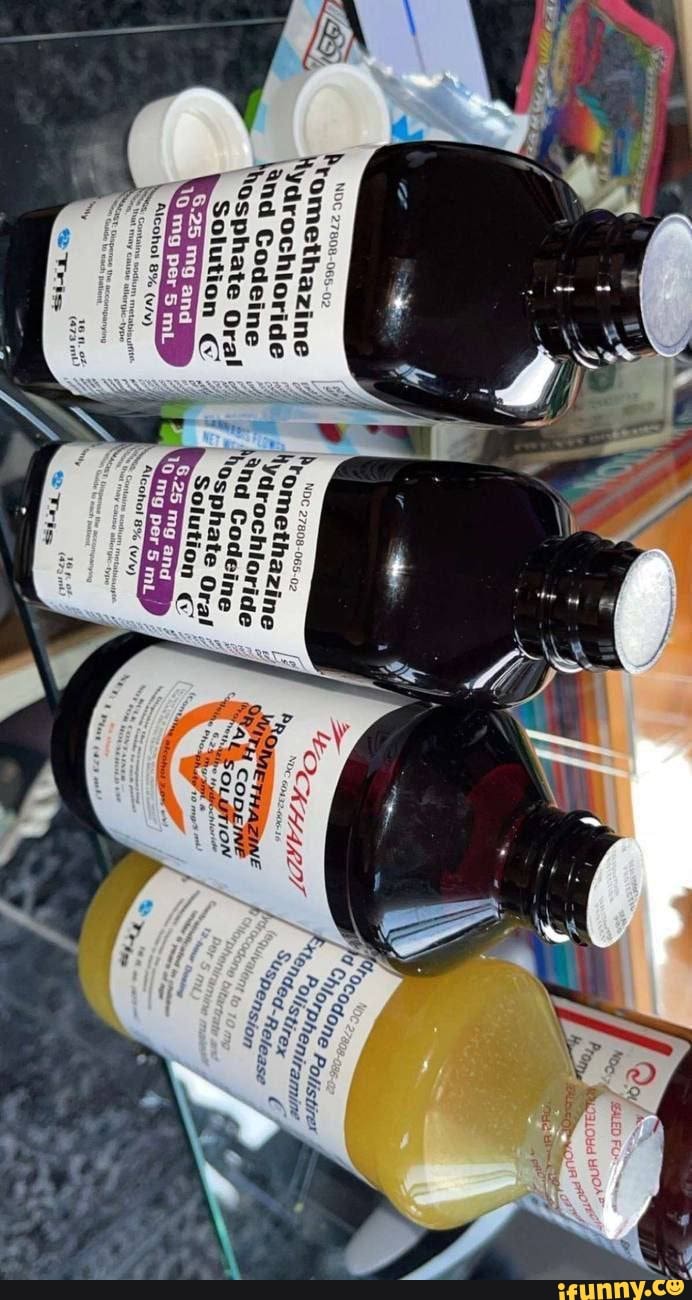
F - National Drug Code Directory NDC Proprietary Name Non Proprietary Name Labeler Name; 77347-001: F Matic Alcohol Based Hand Sanitizer: ... FACE IT HD PERFECT BB SPF30 PA 02: THEFACESHOP CO., LTD: 51523-111: FACE IT OIL CUT DUAL BB EMULSION SPF20: THEFACESHOP CO., LTD: ... 27808-206: Febuxostat: febuxostat: Tris Pharma Inc: 27808-207: Febuxostat: febuxostat: Tris Pharma Inc ... All4car.ru Ассортимент Ford Интернет магазин автозапчастей. Поставка оригинальных и неоригинальных запчастей из Эмиратов (ОАЭ), Японии, Европы, Америки и Китая. NDC 27808-065-02 Promethazine ydrochloride and Codeine Osphate Oral ... NDC 27808-065-02 Promethazine ydrochloride and Codeine Osphate Oral Solution 6.25 mg and Alcohol 8% (wAy) Contains sodium metabisuifis nte that may cause allergic-type Orspense the accompanying "ton Guide to aack patent. 4, 16 only
Ndc 27808-065-02. NDC 27808-065 Promethazine Hydrochloride and Codeine Phosphate NDC 27808-065 is National Drug Code (NDC) product code of Promethazine Hydrochloride and Codeine Phosphate. Detailed data about this drug was submitted to The US Food and Drug Administration (FDA)'s database by Tris Pharma Inc. What is the information related to NDC 27808-065 and what are their meanings? This post will provide following ... Vail Health | Compassionate Care, Exceptional Outcomes LINE TYPE,CHARGE CODE/ PACKAGE,CHARGE DESCRIPTION,DRG,CPT?,HCPCS,MODIFIER,REV CODE,NDC,GROSS CHARGES,SELF PAY CASH PRICE CDM,4930504,DEFIBRILLATOR IMPLANTABLE COBALT ... NDC Code 27808-084-02 - Morphine Sulfate | pharmacompass.com PharmaCompass the one-stop, pharmaceutical information platform accelerates generic drug development by sharing the list of inactive ingredients used to develop Morphine Sulfate marketed by Tris Pharma Inc under NDC Code 27808-084-02 containing the following excipients ANHYDROUS CITRIC ACID(unii: XF417D3PSL), CITRIC ACID MONOHYDRATE(unii: 2968PHW8QP), EDETATE CALCIUM DISODIUM(unii: 25IH6R4SGF ... 27808-065-02 | Promethazine Hydrochloride And Codeine Phosphate ... NDC Package Code: 27808-065-02: The labeler code, product code, and package code segments of the National Drug Code number, separated by hyphens. Asterisks are no longer used or included within the product and package code segments to indicate certain configurations of the NDC. Package Description: 473 ML IN 1 BOTTLE, PLASTIC (27808-065-02)
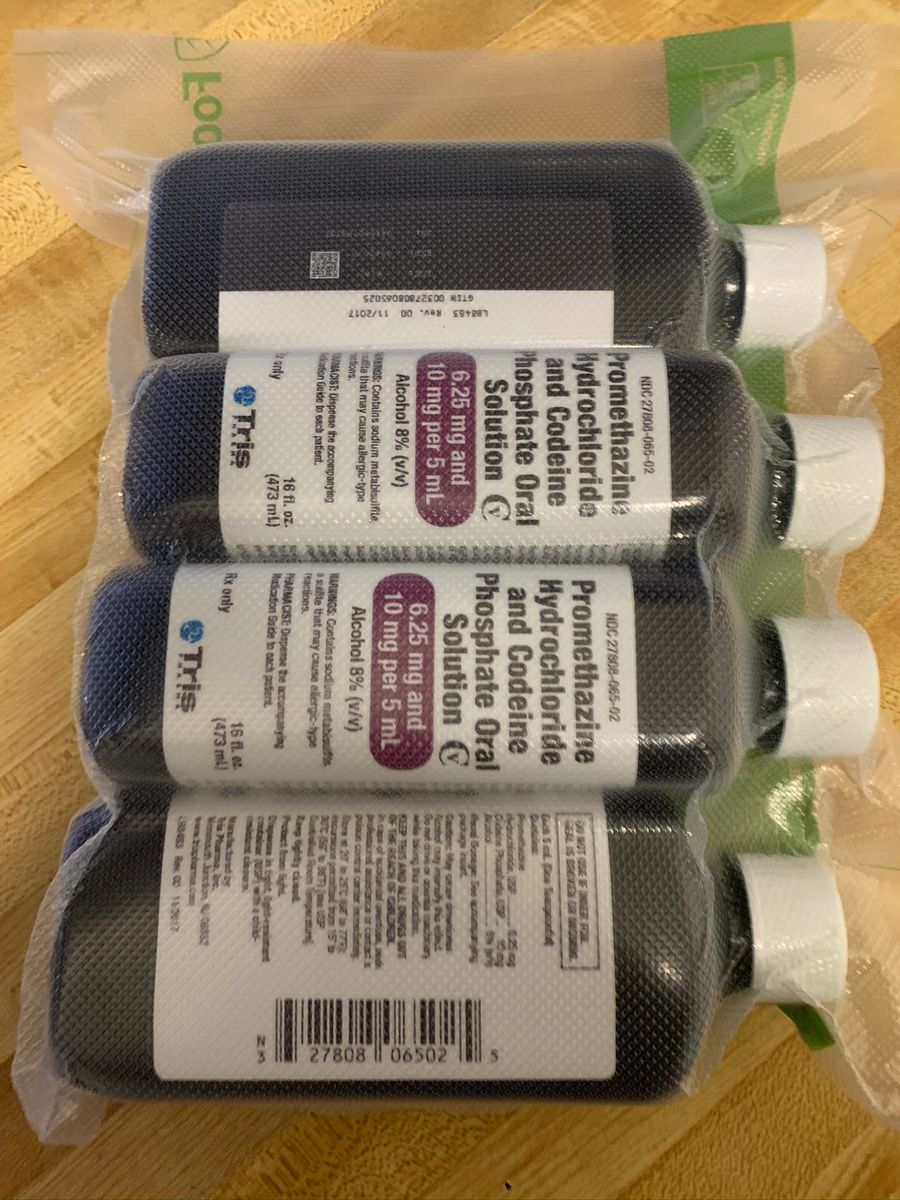
G 035 Pill (White/Capsule-shape) - Pill Identifier - Drugs.com NDC Code Labeler / Repackager; 27808-0035 50474-0930 (Discontinued) UCB, Inc. Drug Uses Add to Drug List Print. Get help with Imprint Code FAQs. Related images for "G 035" Lortab 5/325. Spectrobid. Glucophage. More about acetaminophen / hydrocodone ... DailyMed - Search Results for promethazine NDC Code(s): 50383-803-04, 50383-803-08, 50383-803-16 Packager: Hi-Tech Pharmacal Co., Inc. PROMETHAZINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE ORAL SOLUTION solution Promethazine Hydrochloride and Codeine Phosphate (Page 11 of 13) Bottles of 16 fluid ounces (473 mL), NDC 27808-065-02. Keep bottles tightly closed. Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.] Protect from light. Dispense in tight, light-resistant container (USP/NF) with a child-resistant closure. NDC 27808-065-02 HUMAN PRESCRIPTION DRUG - HIPAASpace The NDC Code 27808-065-02 is assigned to "Promethazine Hydrochloride And Codeine Phosphate " (also known as: "Promethazine Hydrochloride And Codeine Phosphate"), a human prescription drug labeled by "Tris Pharma Inc". The product's dosage form is solution, and is administered via oral form. Additionally
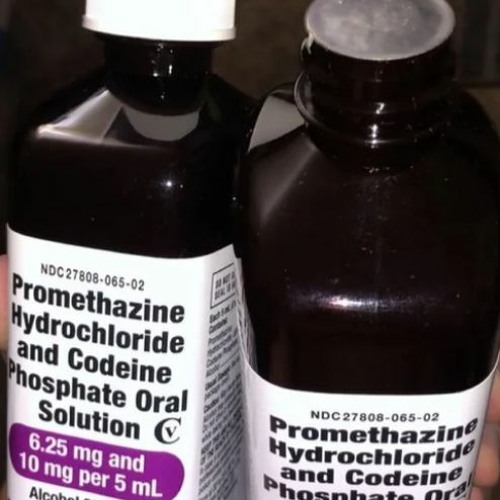
NDC 27808-065 Oral Solution Promethazine Hydrochloride and Codeine ... Promethazine Hydrochloride and Codeine Phosphate is a Oral Solution in the Human Prescription Drug category. It is labeled and distributed by Tris Pharma Inc. The primary component is Promethazine Hydrochloride; Codeine Phosphate. Packaging NDC 27808-065-02 473 mL in 1 BOTTLE, PLASTIC (27808-065-02) NDC SPL Data Element Entries Promethazine Hydrochloride and Codeine Phosphate (Tris Pharma Inc): FDA ... NDC 27808-065-02. Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV. 6.25 mg and 10 mg per 5 mL. Alcohol 8% (v/v) ... NDC:27808-065-01: 237 mL in 1 BOTTLE, PLASTIC: None: 2: NDC:27808-065-02: 473 mL in 1 BOTTLE, PLASTIC: None: Marketing Information: Marketing Category: Application Number or Monograph Citation: NDC Code 27808-065-02 - Codeine Phosphate | pharmacompass.com PharmaCompass the one-stop, pharmaceutical information platform accelerates generic drug development by sharing the list of inactive ingredients used to develop Codeine Phosphate marketed by Tris Pharma Inc under NDC Code 27808-065-02 containing the following excipients ALCOHOL(unii: 3K9958V90M), ANHYDROUS CITRIC ACID(unii: XF417D3PSL), ASCORBIC ACID(unii: PQ6CK8PD0R), D&C RED NO. 33(unii ... Tris Pharma Inc FDA Filings 2022-03-02: Dextroamphetamine. Tris Pharma Inc. Prescription Medication: 2022-03-02: DOXYCYCLINE HYCLATE. Tris Pharma Inc, Changzhou Pharmaceutical Factory. Prescription Medication: ... NDC 27808-065. Tris Pharma Inc. Promethazine Hydrochloride And Codeine Phosphate: 2018-03-12: NDC 27808-158. Tris Pharma Inc. Rosuvastatin Calcium: 2018-01-08 ...
Promethazine memes. Best Collection of funny Promethazine pictures on ... and Codeine I Phosphate Oral hate Oral Phosp'! C Solution Solution @ NOC: 27808-065-02 NDC 27808-065-02 Promethazine Promethazine Hydrochloride Hydrochloride 'Alcohol 8% Alcohol 8% / coin souy mg and 10 mg per mL tat may eate aege-tpe sons ols tt Guide to ech patent Nonty (473m)
ALL4CAR Ассортимент. Мы поставляем оригинальные и неоригинальные детали от ведущих производителей из ОАЭ, Германии, Америки, Кореи и Японии. Все номера, как правило, кроссуются и отражаются в прайс ...
12634-907 : Promethazine Hydrochloride and Codeine Phosphate Oral Syrup 27808-065 Promethazine Hydrochloride and Codeine Phosphate Oral Syrup by Tris Pharma Inc 50090-1482 Promethazine Hydrochloride and Codeine Phosphate Oral Syrup by A-s Medication Solutions LLC 50383-804 Promethazine Hydrochloride and Codeine Phosphate Oral Syrup by Hi-tech Pharmacal Co., Inc.
NDC Ingredient Name NDC Company Name Application Number or Regulatory Citation Product Type Marketing Category CODEINE PHOSPHATE; PROMETHAZINE HYDROCHLORIDE: 12634-909-06 ... 27808-065-02 : Tris Pharma Inc : ANDA200386 : HUMAN PRESCRIPTION DRUG : ANDA : CODEINE PHOSPHATE; PROMETHAZINE HYDROCHLORIDE: 52959-118-04 : H.J. Harkins Company,Inc.
Mercy Health | The Highest Quality Care across Ohio & Kentucky [ { "Code" : "", "Procedure Description" : "Bilateral Ear Lobe Repair", "NDC" : "", "Rev Code" : "", "IP Price" : 750.0, "OP Price" : 750.0, "" : 450.0, "BCBS [3123 ...
View our high-quality generics | Tris Pharma The Tris Pharma Generics division is focused on creating high-quality, patient-friendly products that leverage our strengths in product selection, product development, commercial launch, and securing market share. Driven by innovation, we are working to bring to market 20+ generic products in our pipeline and transform new ideas into effective ...
NDC 27808-065-02 Promethazine Hydrochloride And Codeine Phosphate The NDC Code 27808-065-02 is assigned to a package of 473 ml in 1 bottle, plastic of Promethazine Hydrochloride And Codeine Phosphate, a human prescription drug labeled by Tris Pharma Inc. The product's dosage form is solution and is administered via oral form. NDC Code Structure 27808 - Tris Pharma Inc
Metformin Recall 2018 | DiabetesTalk.Net shakiness dizziness or lightheadedness sweating nervousness or irritability sudden changes in behavior or mood headache numbness or tingling around the mouth weakness pale skin hunger clumsy or jerky movements if hypoglycemia is left untreated, severe symptoms may develop: confusion seizures loss of consciousness call your doctor immediately if …
PROMETHAZINE HYDROCHLORIDE AND CODEINE PHOSPHATE solution - DailyMed NDC 27808-065-02 - Promethazine Hydrochloride and Codeine Phosphate Oral Solution CV - 6.25 mg and 10 mg per 5 mL - Alcohol 8% (v/v) WARNINGS: Contains sodium metabisulfite, a sulfite that may cause ... INGREDIENTS AND APPEARANCE Product Information View All Sections Find additional resources (also available in the left menu) Safety
NDC 27808-065-02 Promethazine ydrochloride and Codeine Osphate Oral ... NDC 27808-065-02 Promethazine ydrochloride and Codeine Osphate Oral Solution 6.25 mg and Alcohol 8% (wAy) Contains sodium metabisuifis nte that may cause allergic-type Orspense the accompanying "ton Guide to aack patent. 4, 16 only
All4car.ru Ассортимент Ford Интернет магазин автозапчастей. Поставка оригинальных и неоригинальных запчастей из Эмиратов (ОАЭ), Японии, Европы, Америки и Китая.
F - National Drug Code Directory NDC Proprietary Name Non Proprietary Name Labeler Name; 77347-001: F Matic Alcohol Based Hand Sanitizer: ... FACE IT HD PERFECT BB SPF30 PA 02: THEFACESHOP CO., LTD: 51523-111: FACE IT OIL CUT DUAL BB EMULSION SPF20: THEFACESHOP CO., LTD: ... 27808-206: Febuxostat: febuxostat: Tris Pharma Inc: 27808-207: Febuxostat: febuxostat: Tris Pharma Inc ...


































Post a Comment for "42 ndc 27808-065-02"